
The Future of Batteries
…and the Future of Energy
Batteries power the clean energy transition, but current technology is falling short. Breakthroughs in chemistry, design, and policy will decide how quickly better batteries arrive, and how much they reshape our energy future.
As the world stumbles toward a low-carbon future, batteries provide both hope and hazard. They are critical to the two most transformative advances: electric vehicles (EVs), and renewable power. But the best batteries available are not good enough to produce better electric cars or to store more energy for the electric grid. New technologies are needed to produce the right combination of power, lifetime, size, cost, and safety. Whether powering an electric car or backing up solar panels and wind turbines, the next developments in the energy revolution require a new generation of battery technology.
Lithium-ion batteries, first brought to market by Sony in the 1990s, led to the widespread adoption of handheld electronic devices such as cell phones and portable computers. In the years following, the price of lithium-ion batteries dropped by 97% and their performance tripled. These advances changed electric vehicles from a niche market to a global industry. Growth in the sales of EVs has outpaced gasoline-powered cars ever since.
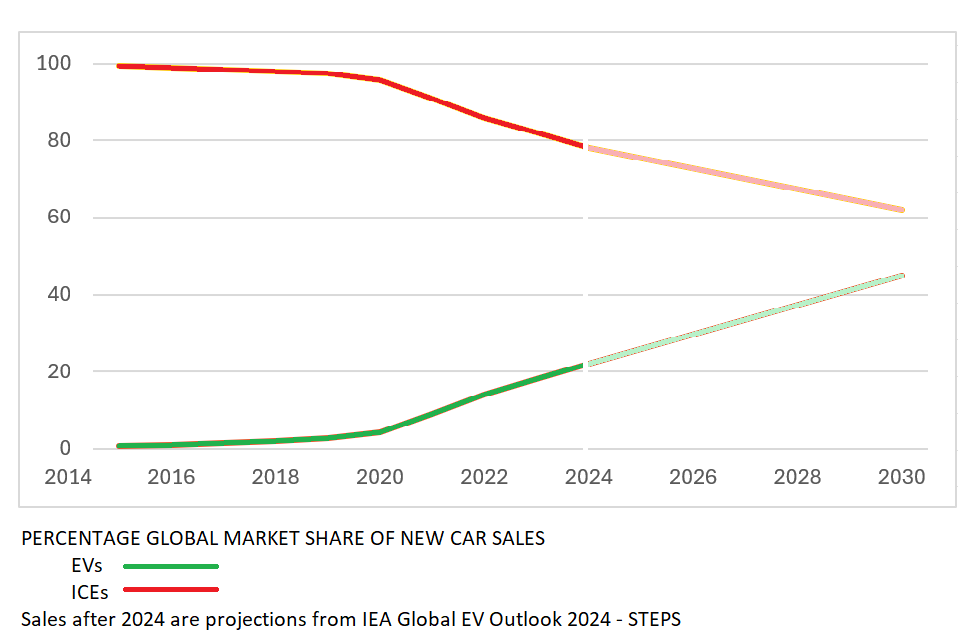
But now lithium-ion (Li-ion) technology is approaching its physical limits. A kilogram of the best lithium-ion battery will hold about 300 Watt-hours of energy. A kilogram of gasoline powering an efficient car engine provides about 3,200 Watt-hours, more than ten times as much. The next generation of batteries must have new chemistry to truly replace gas-powered engines.
The Chemistry of Power
To understand new battery technologies, it’s helpful to know something about how batteries work. Electrons orbit around an atom in distinct layers called shells, similar to the layers in an onion. When a shell has exactly the number of electrons it can fit—fewer in the inner layers, more in the outer—it’s full, an ideal state. The atoms of some metals, like lithium, have only a few electrons in their outermost shell and surrender them easily so that the shell below is full. The atoms of some other materials, non-metals, are almost full in their outer shell, perhaps missing just one or two electrons.
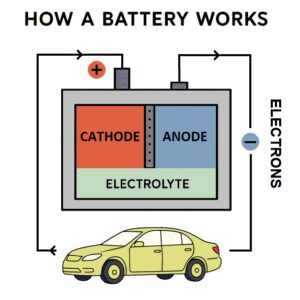
When these materials are brought together the electrons move energetically from the metal to the non-metal. This basic idea underlies how batteries work. A battery contains a donor metal on the “anode” side and a receptor non-metal on the “cathode” side and these are kept insulated from each other. The electrons can flow from the anode to the cathode when an external wire connects them. This flow is the energy that can power a mobile phone or a flashlight or an electric vehicle. It’s available until the metal runs out of electrons to donate or the cathode material becomes full. Then the battery is “dead,” and must be replaced or recharged (forcing the electrons back to their starting point).
There are also physical requirements for how the electric charge must be balanced inside the battery. These are met by including an electrolyte—a substance that plays a somewhat similar role in your body. The electrolyte, which in a battery is often a liquid acid, interacts with both the cathode and anode to keep the battery electrically balanced. The electrolytes used in lithium-ion batteries are flammable and are a safety hazard if the battery becomes defective. Battery research is often directed at finding new materials for cathodes and electrolytes.
Lighter, Safer, and More Powerful
EV batteries need to store the most energy in the smallest and lightest package, known as “energy density.” New technologies are competing to improve this property over conventional Li-ion. Solid-state batteries are the most likely upgrade to be first to market. These are still based on Li-ion chemistry but replace the liquid or gel electrolyte with a solid, often a kind of ceramic or plastic. Solid-state batteries have shown the potential to increase charge density by 50% or more.
Crucially, solid-state electrolytes are non-flammable. In addition to improving safety, this should allow for faster charging times. Solid-state batteries are still expensive and difficult to manufacture but breakthroughs are happening quickly, and they are likely to appear in new EVs before the end of the decade.
Sodium-ion (Na-ion) batteries aim to replace lithium altogether. Sodium—cheap and abundant—essentially replaces lithium in the same battery design. Sodium-ion batteries have low energy density and provide shorter range between EV charging, but they are far cheaper and less damaging to the environment than mining lithium. Na-ion batteries are already appearing in low-cost EVs in China, where short-range urban driving is common and price matters more than range.

More exotic innovations are being pursued in the lab. Lithium-sulphur and lithium-metal batteries could offer dramatic gains in energy density, making longer-range EVs more practical. But they are expensive and so far have safety and durability issues. These chemistries still include lithium, so they continue to carry the environmental cost of lithium mining. Future developments could entirely remove this concern as well as lower costs dramatically. One such process under study is extracting electrical energy from the rusting of iron.
The Grid Must Grow
Safety, greater energy density, more time before recharging and faster recharging times—all these qualities are important, but aside from safety, which ones matter most depends on how the battery is used. Batteries for grid storage prioritize lower cost, maximum number of allowed recharges, improved lifespan, lower environmental impact and easier recycling. The high energy density required for EVs is less important.
EVs get the headlines, but batteries for storing electricity for the grid are a more urgent need. An astonishing 2.6 terawatts of clean-energy projects in the US—twice the existing installed base of wind and solar power, and roughly a quarter of the world’s entire electricity generation capacity—is sitting idle waiting to plug into the grid. Batteries not only compensate for the intermittency of solar and wind, they also reduce the strain on transmission infrastructure and improve grid reliability.
At present, the most common method of storing grid energy is “pumped hydro” —when excess electricity is available, it is used to pump water back up into a reservoir. The extra water is later used for hydroelectric power when solar or wind are offline. Clearly though, this method is not available everywhere, and new battery storage is desperately needed, especially in the sites best suited for solar power. Grid storage requirements in remote locations make Na-ion technology very appealing.
Here, the low energy density of Na-ion batteries is not a major issue. Utilities can simply put many big, cheap batteries in an empty lot near solar panels or wind turbines. These same considerations allow for a wide variety of batteries that would never fit in a vehicle, such as redox flow batteries—giant vats containing metal floating in water and large tanks of electrolyte. Dozens of different types of these are under development. Redox flow batteries also can discharge and recharge thousands of times before needing to be replaced.
Roadblocks—Regulatory and Political
Despite obstructions from the fossil fuel industry, EVs are set to dominate. Better batteries are coming, likely first solid-state and eventually replacements for Li-ion. Before very long, consumers in the world outside of the US will be able to choose between affordable city cars with modest range and premium models with top-tier performance. Charging infrastructure is expanding and charge times are falling. Commercial fleets of delivery vans and farm vehicles are already converting. Long-haul trucks will follow.
The future of grid storage is less certain. Obtaining approval in the US and elsewhere is a regulatory nightmare with overlapping and sometimes competing jurisdictions. Many projects are stalled indefinitely, and most of these stalled projects, all financed and many completely built, will be canceled before they deliver a single Watt—at present in the US, 80% are abandoned while waiting for approval. It is often cheaper to give up on a project than to wait the average four to five years to connect to the grid.
Opposition to these projects often comes from local environmental groups unwilling to negotiate improvements. The backlog of grid connectivity projects in the US includes over a terawatt of proposed energy storage, about equal to the entire national power capacity. Without urgent reform of the approval process, clean energy production is at risk of becoming a tragedy of missed opportunities.
The future of battery technology is shaped not just by lab breakthroughs but by politics. In the US, the present government is eliminating EV incentives and cutting dozens of energy‑storage programs. Meanwhile, mirroring its growth in renewable energy, China’s battery industry is advancing rapidly. It already produces over two‑thirds of the world’s supply and has imposed export controls on battery technology to protect its lead. American factories had started to close the gap—especially in Na‑ion batteries and recycling innovations—but with the retreat by the US, it now seems certain that the next successes for the clean energy transition will come from China.
